Robert A. Brown, BS, RRT, RPFT, FAARC
Technologist-Driven Protocols (also known as “Patient-Focused Pulmonary Diagnostic Assessment Plans “) may provide a roadmap for decision making, on the flow of Pulmonary Diagnostic testing, based upon the client’s Diagnosis, Medical History, and/or from initial Pulmonary Diagnostic data. Technologist-Driven Protocols (TDPs) may be developed from a combination of evidence-based information and vast clinical and/or laboratory experience. TDPs that are well-designed, and validated, add value to your facility and to your customers (internal and external) by reducing procedure variability and by improving the efficiency, consistency, and the quality of delivered services. They can be focused on commonly performed pulmonary diagnostic procedures, for bronchial provocation test selection, CPET, etc.
TDPs: Why Now?
The healthcare environment has been in a state of transformation for numerous years. Delivery of care costs have dramatically increased without foreseeable substantial abatement (Healthcare costs relative to Gross Domestic Product: 1960 = ~5%; 2021 = 18.5%). Additionally, the need for quality patient interventions (the transition from volume-based to value-based care) along with provider and customer satisfaction are some of the key drivers for change (think “Quadruple Aim”).
Professional healthcare organizations (e.g., ATS, ERS, AARC) currently provide evidence-based information on how to improve the delivery of care. The ERS/ATS combined recommendations/standards, as well as the ATS Pulmonary Function Laboratory Management and Procedure Manual, are excellent resources of evidence-based information for the Pulmonary Diagnostic Laboratory. This evidence-based information can serve as the basis of supporting documentation when developing laboratory specific Technologist-Driven Protocols.
Also, since the beginning of the 21st century, there has been a fundamental explosion of reporting in the quantity and quality of dissemination and implementation research. Numerous conceptual frameworks have been developed and key constructs have been validated in the healthcare arena. Understanding how certain Implementation Science concepts and strategies can be successfully employed, in the TDP development, monitoring, validation, and implementation stages, will help to assure program sustainability.
Where to Begin?
Timing is everything. Therefore, before investing a lot of resources and energy into developing TDPs, it’s probably best to pause and assess your organization’s willingness to be open to change. Think how open to change your organization was during the height of the SARs-CoV-2 pandemic?
Planning considerations:
- Gather supporting evidence-based information and assess various Implementation Science concepts which could help guide the TDP process; from initial plan to full implementation.
- Develop a compelling outline focusing on the potential benefits of implementing TDPs and presenting this information to organizational key decision makers. Be patient, as theses decision makers may have competing priorities and not appreciate your eagerness to begin this project.
- Solicit early input from laboratory co-workers. Doing so allows them to have a clear understanding of the project intentions, to be part of the development process and hopefully have early buy-in of the project.
- Data suggests that quality initiatives are generally more successful if they are developed using a team-based approach versus a top-down approach. The rationale is that the more understanding that decision makers have regarding the development and implementation of TDPs, the more likely that they will be supportive of the initiative.
- The following includes some key organization TDP stakeholders
- Administration:Understands the intentions of the project; to elevate the quality of delivered services and potentially reduce institutional costs
- Pulmonary Diagnostic Laboratory Medical Director: The laboratory ambassador who will support the initiative with other HCPs. Will also be the go-to person for potential MD 1-on-1 discussions if there is push back.
- Department of Quality:Has a good understanding of process development and implementation.
- Nursing:Representation from Pulmonary Specialty Outpatient Clinic as they may need to interface with potential clients and provide a brief description of Pulmonary Diagnostic Laboratory testing procedures.
- Pulmonary Diagnostic Technologists (PDTs):Absolutely necessary to be active participants, of the entire process, as they are the ones who will be the TDP implementers and ultimately the end-users.
- Develop a draft document, which specifically outlines TDP actions, that are evidence-based and referenced as such. In the absence of evidence-based information, then expert opinion may be substituted, with that limitation being recognized.
- Now is the time when an Implementation Science process should be incorporated, into the planning process, which focuses on designing, monitoring, revising and validating the TDP. The purpose is to assure that a sustainable program has been developed.
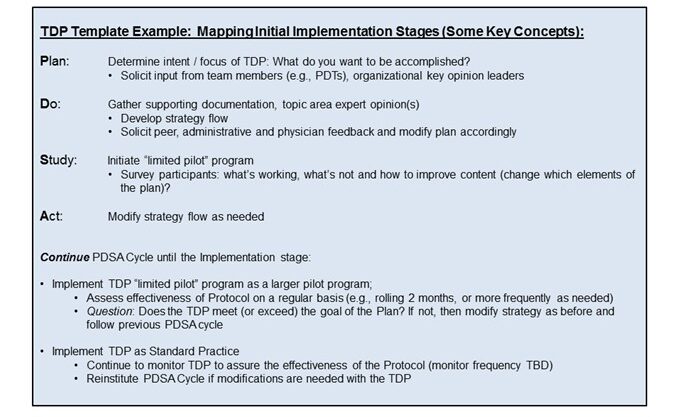
- There are several Implementation Science strategies that may be utilized when designing, evaluating, and implementing a specific TDP. Diagnostic Laboratories commonly incorporate the Plan-Do-Study-Act (PDSA) Cycle as part of the quality process.
- Oftentimes a branching type flowchart is used, as a TDP quick visual aid, to assist the implementer when deciding which test, or sequence of tests, the client should perform.
- This flowchart should be considered as the last step, as it is imperative to provide the rationale behind TDP development. Simply developing a flowchart, without supporting documentation, is only part of the picture and subject to criticism regarding the validity of the TDP.
- Now is the time when an Implementation Science process should be incorporated, into the planning process, which focuses on designing, monitoring, revising and validating the TDP. The purpose is to assure that a sustainable program has been developed.
- The following includes some key organization TDP stakeholders
Evidence-based practices are rapidly becoming the standard for delivering high quality healthcare. By design, Technologist-Driven Protocols are also consistent with this practice.
