INTRODUCING
Vivatmo pro for professional use in clinics and practices!
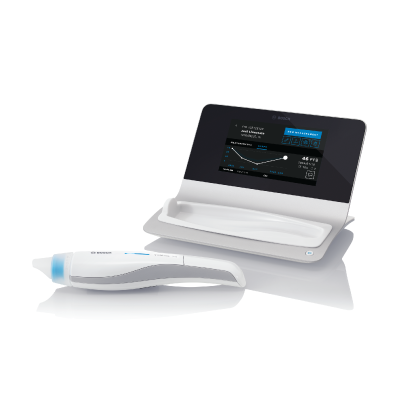
Methapharm Respiratory has partnered with Bosch Heathcare Solutions to extend our portfolio of diagnostic product offerings. Vivatmo pro is an innovative device that measures the level of inflammatory marker in exhaled breath, the fractional exhaled nitric oxide (FeNO). FeNO is an increasingly important marker for airway inflammation that is used in asthma management to support diagnoses and individualized therapy. Providing physicians with an enhanced ability to assess airway inflammation can help ensure that patients receive the medication and treatment needed. The award-winning Vivatmo pro FeNO monitoring device is non-invasive, easy to use, and virtually maintenance-free, utilizing high-precision Bosch sensor technology. Vivatmo pro is suitable for patients aged 7 and older.
TIME TO UPGRADE!


Benefits of FeNO Monitoring
DIAGNOSIS
Assess current level of airway inflammation
MANAGEMENT
Identify patient’s likelihood of corticosteroid responsiveness
Optimize adherence to corticosteroid treatment to aid in reducing exacerbations
Determine appropriate biologic therapy as indicated

How Vivatmo pro works
This animated video explains how Vivatmo pro works.
Vivatmo pro benefits
No maintenance: Bosch engineering excellence
No recalibration, maintenance, or sensor exchange during the entire lifetime (up to 5000 measurement trials) due to high-precision Bosch sensor technology.
Usability: intuitive and easy
- Patient exhales against light pressure through the mouthpiece into the handheld.
- Two different visualizations guiding users through the measurement maneuver.
- Immediate results allow for optimum integration into practice workflows.
- If first attempt fails, Vivatmo pro is ready to measure again within 10 seconds.
- Winner of the Red Dot Design Award.
Connectivity: smart and secure
- Remote software updates available via Vivasuite connectivity solution.
- Common interfaces and data exchange standards enable seamless IT integration: Wifi, USB, Ethernet, HL7, and GDT.
- Patient data management system of up to 10,000 patient files, including export function and immediate printing.
- Backups and optional user login to support data security in clinical environment.
- Portable handheld can perform stand-alone measurements when not paired to basestation.
Hygiene: highest standards
- Reduced infection risk due to easy cleaning, no inhalation through device, and highly efficient germ filter in disposable mouthpiece.
- Cleaning with alcohol disinfectant wipes (max. 30%).
- Bacterial filtration efficiency (BFE): 99.998%.
- Viral filtration efficiency (VFE): 99.960%.
Sustainability: CO2 neutral
The entire Vivatmo system is climate-neutral through carbon offsets.
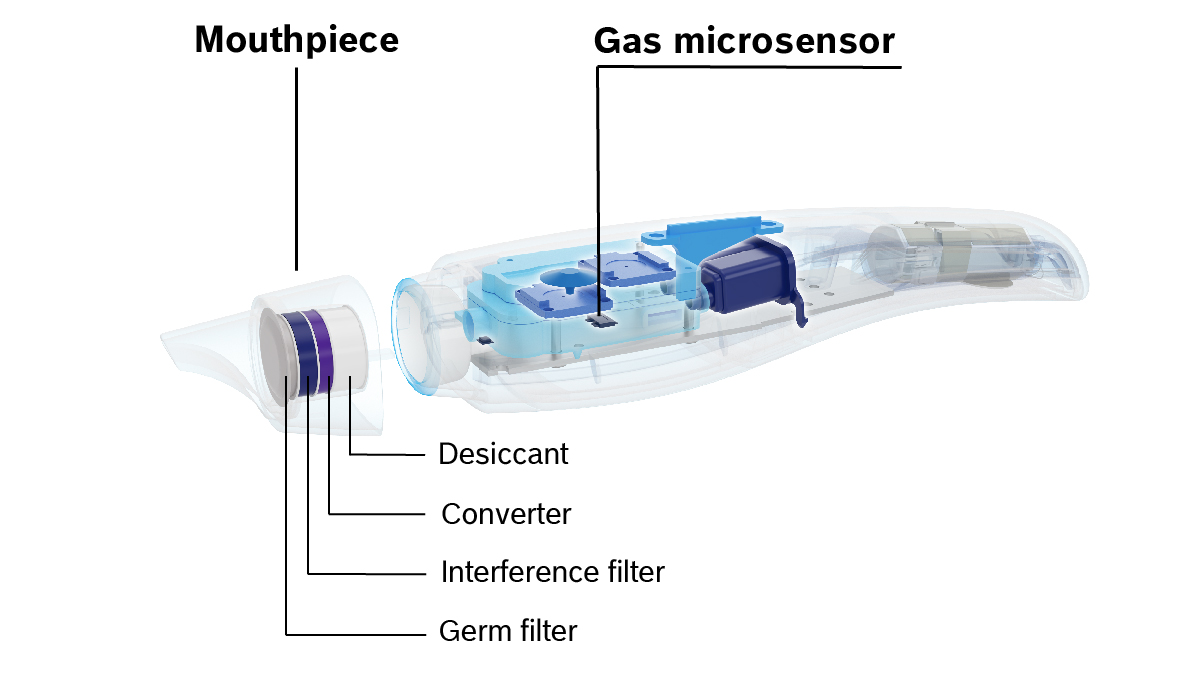
Vivatmo oxycap for optimal hygiene
The Vivatmo oxycap is a high-quality disposable mouthpiece. It contains several complex filter layers. These reliably trap unwanted particles, bacteria, viruses and moisture before the breath reaches the measuring device.
In this way, the mouthpiece optimally prepares the breath sample for the measurement process and ensures that the Vivatmo device remains clean and hygienic inside (BFE/VFE > 99.9%).
Interested in Vivatmo pro?
Contact us – we are happy to support you!
Product Information
Special Conditions for Use Statements
Regardless of displayed measured results, monitor signs or symptoms of chest tightness, shortness of breath, coughing or wheezing for decision about treatment. Do not use the device for subjects suffering from acute upper or lower respiratory infection disease or with current serious medical conditions (other than asthma).
Vivatmo pro should not be used in critical care, emergency care or in anesthesiology. The following conditions can influence correct measurement of results and shall be avoided: smoking or tobacco consumption for at least 1 hour before the measurement, eating or drinking at least 1 hour before the measurement, especially nitrate rich food (e. g., spinach), strenuous exercise, use of rescue inhaler or leukotriene modifier 1 h before measurement.
The device is not indicated for children under 7 years of age including infants, or by patients who are unable to understand and execute the instructions given by healthcare providers, as measurement requires patient cooperation.
For prescription use only.
For full instructions and warnings, please refer to Vivatmo pro instructions for use manual.








